The history of the atom... the secrets of the tiny particles that make up our modern world
The sun is not only the primary source of energy and light for our planet, but it dominates with its majestic magnitude over the solar system with all it contains of planets, moons and meteorites.
Contrary to what it seems in the sky of stillness and stability, this huge mass is in a constant state of frenzy and eruption represented by the huge amount of energy and heat that we notice and feel, which is equivalent to detonating 100 billion tons of dynamite every second, according to the NASA space agency. 1
If we try arbitrarily and suppose to quench this radiant energy, we need a swimming pool of more than 6 trillion square kilometers full of water to pour it on the sun, and if we think that this trick will deceive the sun by virtue of our prior knowledge of the relationship of water and fire, we are probably wrong.
The pattern and concept of fires on Earth is completely different from what is on the Sun, as the processes of heat generation there depend on nuclear fusion, whereby four atoms of hydrogen fuse to produce one atom of helium, in addition to the energy resulting from the fusion process.
The sun will not be affected by any amount of water spilled on it. On the contrary, this will contribute to igniting the sun and providing it with fuel, because water is a chemical compound consisting of two hydrogen atoms and an oxygen atom. When the water evaporates due to the heat, the hydrogen will join the rest of the hydrogen atoms that make up the mass of the sun.
According to Einstein's famous equation, which says that there is a strong relationship between mass and energy, we find that all solar energy emitted results from the interaction of its mass, which is represented by hydrogen atoms by 72%, helium atoms by 26%, and the rest is represented by some atoms of heavier elements such as Oxygen, Carbon, Nitrogen, Magnesium, Iron and Silicon. 2
The era of the atomic current.. The roots of the theory in the ages of the Greek philosophers
Today we find ourselves talking with complete confidence about the chemical composition of the sun, the earth and the rest of the heavenly bodies, and with bold and confident steps we can distinguish between the elements and the atomic difference between each other, and this is nothing but a great work of discovering the atom and conducting various experiments on it, to verify the existence of the atomic model in the universe.
The atomic theory indicates that the universe, with all it contains of matter, consists of very small, indivisible particles or atoms, and the roots of the theory go back to the era before the Greek philosopher “Socrates” in the fifth century BC, and that era witnessed the emergence of the atomic current (Atomism), which was pioneered by the Greek philosopher "Leucippus" and his famous student "Democritus." Some ancient Indian texts also appeared that speak of the same atomic concept of matter dating back to the same era. 3
Despite this, the atomic model and the possibility of the existence of atoms that make up matter in the universe were not considered until the nineteenth century, when the “scientific method” provided an opportunity to observe and experiment with invisible particles, and then opened to man the treasuries of a new world that still brings him information and fascinates him with wonders. Even today.
The theory of atomic structures.. the fruit of chemical reactions of fluids
The English chemist and physicist John Dalton was able to write the first features of the atomic world in an academic and scientific way after conducting several experiments that led to his development of a theory about atomic structures.
Dalton's work in the "Manchester Literary and Philosophical Society" in 1800 resulted in several studies and research on the chemical reactions of fluids, including his famous experiment on a different group of gases, such as oxygen, hydrogen, nitrogen, and others, and calculating the pressure product caused by these gases. combined in one vessel, and comparing them to the total pressure caused by the same gases and the same quantity, but separately; "Dalton" found that the numbers are equal, so he submitted his research paper, talking about his law, "Dalton's Law" of gases.
While working on this experiment, Dalton noticed that there are gases that can only be combined in certain proportions.
Dalton's theory states that a chemical element is made up of indivisible, infinitesimal particles called atoms.
The first is the law of conservation of matter, formulated by the French chemist Antoine Lavoisier in 1789, which states that the total mass in any chemical reaction remains constant, in other words, the mass of the reactants is equal to the mass of the substances produced in a chemical reaction.
The other law is the law of constant proportions. It was first approved by the French chemist "Joseph Louis Proust" in 1799, and it states that any pure compound always contains the same proportions of its compound elements. For example, table salt "sodium chloride", contains the same ratio between the elements sodium and chlorine, regardless of the amount or source of the salt. 4
"Dalton's Theory".. Five revolutionary points that established modern atomic science
The outputs of "Dalton's" theory of the atom were summed up in 5 revolutionary points:
1. “All matter is made of particles called atoms.” Dalton assumed some of the characteristics of these particles, such as hardness and impenetrability, and here the fatal mistake he made becomes apparent, and the reason is understood because the scientific tools did not help him at that time.
2. “Atoms of any given element are identical in mass and properties.” For example, there will be no difference between any two atoms in pure gold, regardless of its quantity and mass, and vice versa as well. The sodium atom is different from the carbon atom. Some elements may share many points such as boiling point, melting point, and electrical conductivity, but the atoms of any two different elements will not be identical.
3. “Atoms of different elements can be distinguished by calculating the atomic weights.”
4. "The atoms of the different elements combine to form multiple chemical compounds, with new and different properties." Table salt, for example, is composed of sodium, which is a highly reactive alkali metal with chlorine, which is a poisonous gas.
5. Finally, "the chemical reaction is nothing but a rearrangement of atoms between each other, and it is not possible in any way to destroy or negate any atom, or even to recreate it and return it to nature." 5
Dalton's conclusions were direct and practical in formulating linear chemical equations, but they lacked access to anything less than the atom itself. In this model, we find it solid and indivisible, and without any electric charge. This misconception was corrected by the British scientist "Ernest Rutherford" in the following century.
"Joseph Thompson" .. The coincidence of discovering particles smaller than the atom
After about 100 years, the British scientist Sir Joseph Thomson made experiments in electricity and radioactivity, which resulted in the discovery of particles smaller than the atom itself, which are electrons, and this confused the calculations at first, but eventually led him to adopt a model New to the atom, instead of being a solid, rigid body, "Thomson" indicated that there is a group of negatively charged electrons swimming in the vicinity of a positively charged atom.
In 1897, Thomson conducted an experiment in which he noticed that cathode rays - a stream of rays affected by magnetic and electric fields - consist of unknown, negatively charged particles. He soon concluded that these particles should be much smaller than an atom, and that the value of its charge is directly proportional to its mass.
Thomson initially called these particles "fine particles", and he noticed that they could be extracted from any material, and their name was later changed to what we know today as electrons and singularly electron. 6
Thomson received the Nobel Prize in Physics 9 years after his discovery. With a positive charge in which the negatively charged electrons swim, then valence occurs. This model was likened to the English Plum Pudding in an attempt to simplify.6
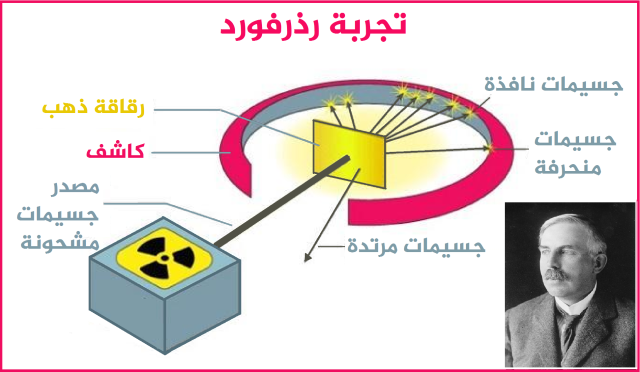
Ernest Rutherford: The gold foil experiment that produced the nucleus of an atom
Despite the dramatic shift in scenery, Thomson's model did not hold up much with the emergence of New Zealand star Ernest Rutherford, one of Thomson's former students, after he conducted the famous Gold Foil Experiment, which resulted in the discovery of a nucleus. Corn in 1911.
Rutherford noticed that positive waves do not spread throughout the atom, but rather they are concentrated in a narrow space in the middle, where the nucleus is located, and in light of this, the discovery of the subatomic particle, the proton, appeared, which is completely equal to the charge of the electron, but it is positively charged.
Rutherford's experiment came to reveal the nucleus and its positive charge, and that the atom is not a solid body, as Dalton assumed.
Thus, Rutherford prompted him to restore his teacher's atomic model, after he fired a beam of alpha particles, which are positively charged particles with a mass 7000 times heavier than an electron, on a thin sheet of gold, causing him to be very confused. Rutherford commented then: It was unbelievable, as if you had fired a 15-inch shell at a tissue, and the shell would bounce back and hit you. 7
Rutherford's experiment revealed that most of the alpha particles passed to the other end after penetrating the gold plate, indicating that most of the space inside the atom is empty space. He noticed that some alpha particles were slightly deviated from their path, which may indicate the possibility of a collision between them and other positive particles found with them in the same atom. While there were alpha particles that deflected greatly, a few of them bounced back towards the radiation source.
His conclusion was that this does not happen unless there is a huge repulsive force that results in this deflection, and to achieve this force this positive charge must be localized at a point, not spread throughout the atom. As a result, he discovered another subatomic particle, the "proton", which is located inside the nucleus, and carries a positive charge equal to the negative charge of the electron.
Rutherford's model describes the atom as having a positively charged nucleus in the middle, and the size of the nucleus is relatively small, but it is of high density, as most of the mass of the atom is concentrated, and a group of negatively charged electrons revolve around it at certain distances governed by the electrostatic force. Rutherford based his model on the solar system, as he compared the nucleus to the sun, and the electrons are the planets that revolve around it due to the force of gravity. 8
Niels Bohr: A model that satisfies chemistry and opposes quantum physics
This was not the first time that the structure of the atom was likened to the solar system, as several physicists had preceded the same proposal, the most famous of which was the Japanese "Hantaro Nagaoka", but in any case, the "Rutherford" model did not last long as well. Bohr, along with Rutherford himself, worked on some substantial modifications to the earlier model.
The planetary model of the atom had two important flaws. The first was that, unlike planets, electrons were charged particles that were affected by the electromagnetic field. The other problem is that the planetary model of the atom is unable to explain the phenomena of high spectra emission and absorption in atoms.
Bohr's model came to explain the fact that electrons move in their orbits around the nucleus and how they gain or lose energy
It is fortunate for the physicist “Bohr” that his era coincided with the start of work on the theory of quantum physics at the beginning of the twentieth century, which made a great revolution in the natural sciences of humans about how the universe works. The German scientists “Max Planck” and “Albert Einstein” suggested that Light energy is emitted or absorbed in discrete quantities known as quanta. Which appealed to Bohr, to combine this definition with the atom model, which led him to conclude the following three outputs:
1. Electrons revolve around the nucleus of an atom in circular orbits of known energy.
2. The energy of the orbit is related to its distance from the nucleus, so that the lowest energy levels are in the orbits closest to the nucleus, and vice versa.
3. The atom emits or absorbs radiation depending on the movement of the electron and its transfer between orbitals. 9
Of course, Bohr relied on the hydrogen atom, because it is the simplest and lightest atom in the universe, as it contains one proton inside the nucleus, and one electron hovers around it in return in a circular orbit, and in the event that the electron moves from one orbit to another, it either radiates or absorbs energy Electromagnetic. These orbits allow only a limited number of electrons to exist, so that the larger the orbital, the higher the number of allowed electrons.
And it seems that the Bohr model is the closest and simplest model to the truth, and it seemed more than enough for chemists to deal with atoms, it did not gain the confidence of physicists who saw that the model still suffers from gaps that contradict the principles of quantum physics, which is the science that studies the properties of Subatomic particles, the most important of which is the Uncertainty Principle or the Uncertainty Principle, which states that it is not possible to accurately measure the position and momentum of a particle at the same moment, but only the prediction is correct. 10
Cloud model.. The mystery of electron movement reveals the secrets of the nucleus
After the famous Austrian physicist Erwin Schrödinger succeeded Max Planck at Friedrich Wilhelm University in Berlin, he focused his attention on the latest findings of atomic theory, and by virtue of his deep involvement in quantum physics, he sought to transform the atomic model from his concept Classical to its true form in quantum physics.
In 1926, "Schrödinger" presented his research papers to mathematical equations dealing with the probability of finding an electron in a specific location in the atom, and these equations were an input and cornerstone for the atom model in its final form that we know today. Schrödinger explained that the electron does not revolve around the nucleus in a classical physical motion, but that there are several locations that appear in the form of a cloud that surrounds the nucleus of the atom and predicts the location of the electron. The advantage of this cloud is that it allows the existence of the electron in a three-dimensional space. 11
Schrödinger discovers that the electron does not revolve in a single circular orbit, but can be found in a cloud of possibilities
This is without the need to delve into more details that are difficult for non-specialists to go into because they deal with the subatomic world as we explained earlier, which is difficult to imagine or formulate even mathematically.
It did not take long for scientists to reach the third particle of the components of the atom. After the electron and the proton, the neutron was discovered, which is a chargeless particle found in the nucleus of the atom. In this way, scientists were able to establish their final argument about the structure of the atom that we know today, without which technology would not have advanced us and produced what we carry today from the modern electronic industries in all its forms, especially the mobile phone.
Sources:
[1] Choi, Charles (2021). Earth's Star: Facts About the Sun's Age, Size, and History. Retrieved from: https://www.space.com/58-the-sun-formation-facts-and-characteristics.html
[2] Same source.
[3] Pullman, Bernard (1998). The atom in the history of human thought. Oxford University Press, Oxford. p 31
[4] Williams, Matt (2014). What is John Dalton's atomic model? Retrieved from: https://www.universetoday.com/38169/john-daltons-atomic-model/
[5] Website editors (date unknown). Dalton's theory of the atom. Retrieved from: https://www.khanacademy.org/science/chemistry/electronic-structure-of-atoms/history-of-atomic-structure/a/daltons-atomic-theory-version-2
[6] Editors of the site (date unknown). Thomson's Model of Corn - The Plum Pudding Model. Retrieved from: https://www.nuclear-power.com/nuclear-power/reactor-physics/atomic-nuclear-physics/atomic-theory/thomson-model-of-the-atom-plum-pudding-model/
[7] Jogrison, Eric (date unknown). Ruthford model. Retrieved from: https://www.britannica.com/science/Rutherford-model
[8] The same Egypt.
[9] Halmstein, Annie Marie (2020). Explanation of the Bohr model of the atom. Retrieved from: https://www.thoughtco.com/bohr-model-of-the-atom-603815
[10] Website editors (date unknown). Uncertainty principle. Retrieved from: https://www.britannica.com/science/uncertainty-principle
[11] Williams, Matt (2016). What is the cloud model? Retrieved from: https://www.universetoday.com/38282/electron-cloud-model/
https://doc.aljazeera.net/%D8%AA%D9%82%D8%A7%D8%B1%D9%8A%D8%B1/%D8%AA%D8%A7%D8%B1%D9%8A%D8%AE-%D8%A7%D9%84%D8%B0%D8%B1%D8%A9-%D8%A3%D8%B3%D8%B1%D8%A7%D8%B1-%D8%A7%D9%84%D8%AC%D8%B3%D9%8A%D9%85%D8%A7%D8%AA-%D8%A7%D9%84%D8%AF%D9%82%D9%8A%D9%82%D8%A9-%D8%A7%D9%84/?fbclid=IwAR1FoJ9d4a7kzYmt11QP2QPxnXyF9tuWrrdccrki0phmuVxBYYLSOcPCjNA