Scientists discover the mystery of turning the Alhambra in Granada purple

About 800 years ago, the Alhambra was a prominent landmark in the Spanish city of Granada and one of the jewels of Islamic architecture in the twelfth century.
Despite restoration work, its colors appear to be changing. A series of mysterious purple spots have appeared on the plaster covering the palace's ornate vaults known as muqarnas (a distinctive element of Islamic architecture) since the 1990s, baffling experts.
Inside, in the gilded halls of the Alhambra, the colors are slowly changing. After centuries of natural weathering, parts of the palace's golden wings and whitewashed ornate walls are turning a mismatched pale purple, which scientists think they can finally explain.
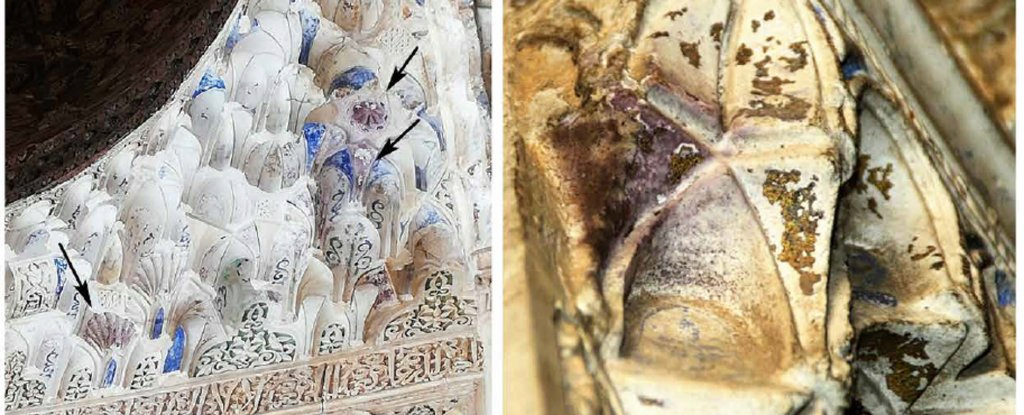
In a new research paper published in the journal Science Advances, mineralogist Carolina Cardel and microscopy specialist Isabel Guerra at the University of Granada explained that after investigating this phenomenon for several years, they finally discovered that the change in the colors of the muqarnas is the product of the erosion of the thin gold sheets that were covered with gold. With a layer of white plaster during the restoration work carried out in the nineteenth century.
They were able to identify the abnormal pattern of heterogeneous gold corrosion, caused by an electrochemical process in which gold dissolves and oxidizes, acquiring a purple color.
Gold is one of the least reactive metals, so it is supposed to withstand the passage of time. This precious metal is resistant to sunlight, moisture, air pollution, and temperatures, which is why it is a valuable material for making jewelry, coins, and, more recently, electronic devices.
Soft and malleable gold was used to decorate palaces, ornaments, weapons, armor, and artwork, using a technique called gilding.
In the case of the Alhambra, the walls of the palace were originally adorned with a thin golden inlay covered with sheets of supple tin. But over time the surfaces turned a strange purple, and soon they were covered with a layer of white plaster in the 19th century.
Turning the warm gold glow to purple is an understandable chemical trick since ancient times, as Roman alchemists used this technique, usually catalyzed with a mixture of nitric acid and hydrochloric acid, known as nitric acid hydrochloride, or “royal water,” to color glass since fourth century.
The “royal water” reaction dissolves the gold in small particles, which dissipate - as the inventor and scientist Michael Faraday suggested in 1856 - the light into ruby red, violet and blue.

So far, no signs of nitric acid hydrochloride have been detected on the walls of the Alhambra.
And without the "royal water" in the mix, a different chemical process had to create a color change inside the Alhambra.
Cardell and Guerra set out to investigate using a scanning electron microscope equipped with an array of spectrometers to reveal the chemical composition of gold-lined Alhambra features down to the nanoscale.
After studying the centuries-old walls of the Alhambra and modeling chemical weathering that may have occurred, the researchers found that an "unexpected set of electrochemical processes" may have shaded damaged surfaces purple.
Cardell and Guerra found gaps and craters in the gold leaf, channels through which moisture can reach and corrode the tin core when the walls are free of dirt.
But where the walls were covered in soot, the gold eroded instead. Stripped of its electrons, the gold gradually decayed and spontaneously formed gold nanoparticles about 70 nanometers in diameter, which, Kardel and Guerra say, are the right size to scatter light waves, making them appear purple.
However, not everyone is convinced that this erosion process led to the discoloration. It's surprising that the gold material turns purple over time, Catherine Lewis, a chemist at the Surface Reaction (LRS) Laboratory in Paris, told APS Physics. But she noted that the scientists had not conducted any experimental tests to try to reproduce the proposed corrosion process.
In their paper, Kardel and Guerra argue that replicating five centuries of natural weathering in lab experiments would be difficult, and would not necessarily produce highly beneficial results.
"Our research was conducted on a real case study of more than five centuries of weathering under natural conditions, which limits our ability to elucidate the exact erosion model," the duo wrote.
Source: websites

