Researchers: Oxygen is not sufficient evidence for the existence of extraterrestrial life
Oxygen became one of the most important gases in Earth's atmosphere more than two billion years ago, and since then most of it has been produced by living organisms in the process of photosynthesis.
Molecules that testify to the presence of life on a particular celestial body are called biomarkers, and traditionally, oxygen is considered one of the best biomarkers.
This is due to the fact that most of it on Earth is formed as a result of the activity of living organisms. However, researchers at the University of Gothenburg in Sweden recently found a new potential abiotic pathway, which is an alternative way to create oxygen from sulfur dioxide.
The press release of the University of Gothenburg, published on October 12, indicates that the sulfur dioxide molecule has been found in the atmosphere of many celestial bodies and large amounts of it can be released into the atmosphere during volcanic eruptions.
Plants absorb carbon dioxide and water and produce sugars and oxygen as a byproduct (Wikimedia)
Oxygen is not evidence of life
It was known that finding oxygen in the atmosphere of an exoplanet is evidence of the existence of life. On Earth, organisms such as plants absorb carbon dioxide, sunlight, and water and produce sugars and starches for energy, and oxygen is a byproduct of this process.
The discovery of oxygen elsewhere outside the globe gives the impression of life, and researchers have long recognized that non-biological or abiotic processes also contribute to the formation of oxygen - particularly in space.
But recently, scientists at the University of Gothenburg discovered a new method that can produce oxygen on exoplanets. Unfortunately, it does not require any kind of life.
According to the University of Gothenburg press release, there is evidence that ionized sulfur dioxide contributes to the formation of oxygen molecules. This may explain the presence of oxygen in the sulfur dioxide-rich atmospheres of many of Jupiter's moons.
Since the element sulfur is not scarce in celestial bodies, since volcanoes produce sulfur and pump it into the atmosphere, volcanic exoplanets may have oxygen in their atmospheres.
?How is oxygen formed in the atmosphere
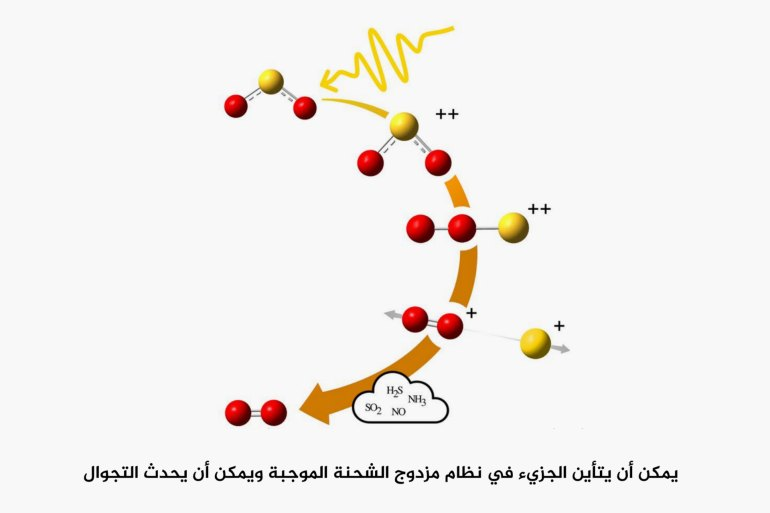
High-energy radiation from stars can ionize a sulfur dioxide molecule, and when the sulfur dioxide ionizes, the molecule rearranges itself into a "positively charged double system".
It can then take a linear form with two oxygen atoms adjacent to each other and a sulfur atom at one end, and the oxygen atoms are free to drift in chaotic orbits until they settle into new compounds.
Sulfur dioxide ionizes when exposed to high-energy radiation (University of Gothenburg)
“When double ionization, two of the bonded electrons in the molecule are expelled and the process leads to changes in the angle between the atoms in the molecule,” says Mance Wallner, PhD student in physics at the University of Gothenburg and first author of the study published in the journal Science Advances. Alternately, as is crucial in the present case, wandering can take place, that is, the atoms switch places, and the molecule assumes a whole new shape.”
Once wandering occurs, the sulfur atom may dissociate, leaving behind a simple positively charged oxygen molecule, which can then be neutralized by receiving an electron from another molecule. This sequence of events could explain how oxygen forms in the atmospheres of many of Jupiter's moons, even though there is no biological life there.
"We also suggest in our article that this occurs naturally on Earth," says Raimund Feifel, a professor at the University of Gothenburg and co-author of the study.
The next step will be to see if oxygen is produced when other molecules, such as carbon diselenide, undergo double ionization. "We want to see if it also happens after that, or if it's just a happy coincidence with the sulfur dioxide," Pfeifel says in the press release.
source:websites